Launch of ADH MASTR� v2
February 17, 2015The new ADH MASTR™ v2 molecular assay is now released for research use only. this assay is an update of the current ADH MASTR™ assay.
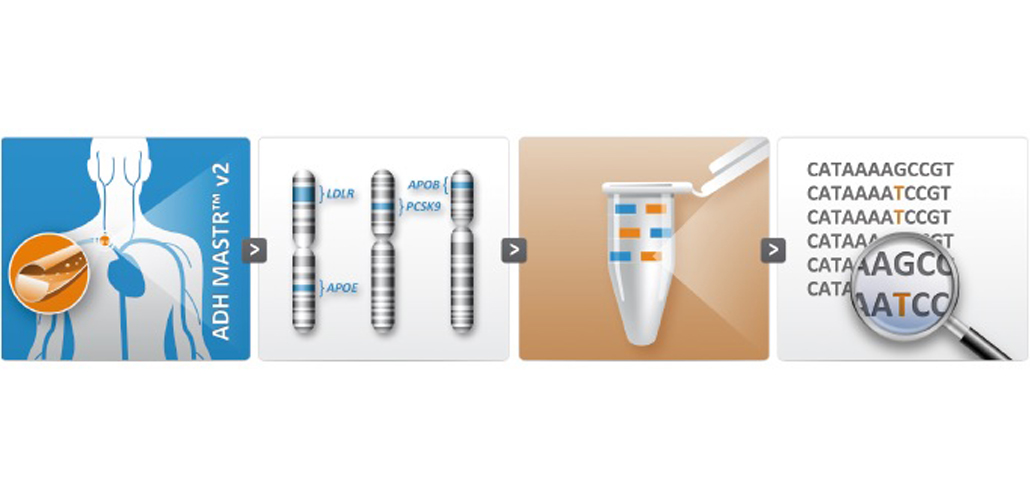
As was the case for the current ADHMASTR™ kit, this new assay is provided as a ready-to-use kit, for the MPS-based identification of all SNVs and CNVs in 3 genes (LDLR, PCSK9, APOE) and part of exon 26 (c.10200 to c.11100) of APOB, underlying monogenic hypercholesterolemia.
Now, in addition, this update assay contains a fifth PCR mix with primers for 12 SNP locations, allowing to study variable penetrance or risk profiling in individuals respectively with or without a monogenic cause.
The other four PCR mixes have not changed. The assay design and workflow protocol is exactly the same as the previous ADH MASTR™, as is the compatibility with MiSeq®, Ion PGMTM and 454TM GS.
The ADH MASTR™ will be fully replaced with the ADH MASTR™ v2.

dv55eRXDrydR9D1sib8D.jpg)
