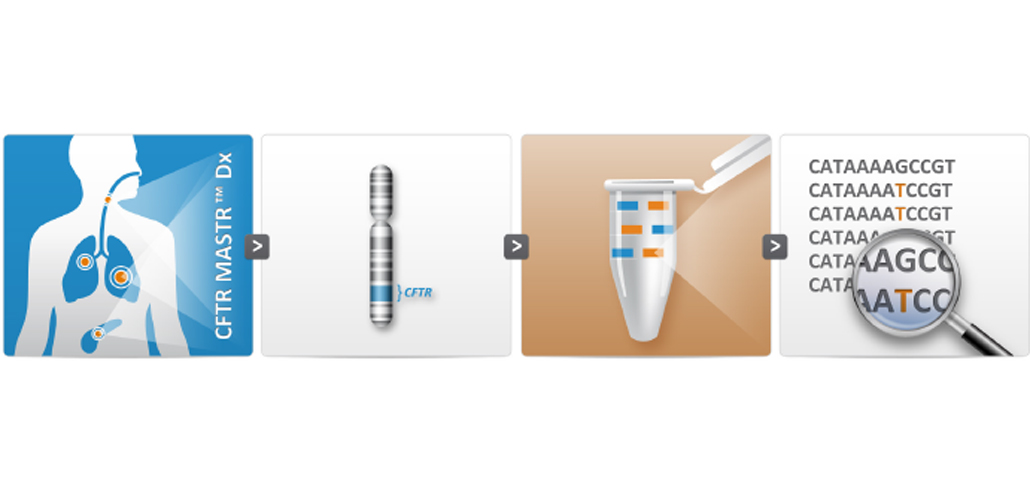
New CFTR MASTR Dx validated for dried blood spots
Anatomy - Pathology / Molecular Diagnostics
March 12, 2015Multiplicom is pleased to announce that CFTR MASTR™ Dx has been successfully validated for an additional sample type: dried blood spots. The CE-IVD claim is now extended to include DNA extracted from dried blood spots, allowing you to use the assay for routine neonatal screening.
The CFTR MASTR™ Dx is a molecular diagnostic assay for identification of sequence variants covering the complete CFTR gene in individuals with increased risk for CF, in CF-carriers or CF-related phenotypes.
Multiplicom’s CFTR MASTR™ Dx assay is provided as a ready-to-use kit that offers robust performance with minimum hands-on time. All reagents necessary to enable multiplex amplification of 48 amplicons (300-450 bp) in two PCR reactions are included, targeting all coding exons, selected intronic regions and part of the promoter region. The assay is validated for DNA derived from whole blood, as well as from dried blood spots.
The assay is compatible with all current Massively Parallel Sequencing (MPS) systems, providing the flexibility to choose your preferred method.